4.3.4 Surface Preparation: Chemical Preparation
A substrate may be perfectly clean and still be difficult to bond. Low surface energy substrates, such as polyolefin plastics, behave like a newly waxed car, making liquids bead up instead of spreading across the surface and clinging. Wettability, the attraction of the surface for an adhesive, may be increased with a chemical primer that raises the substrate’s surface energy, making the resulting adhesive bond much stronger.
Primers act as interfaces between the adhesive and the substrate. They are generally applied as a thin layer in a solvent carrier (1 g/m2) to a low-energy substrate. In some instances, the solvent in the primer may etch the substrate and increase the surface energy/wettability to promote mechanical interlocking (Figure 93). Wetting, diffusion and mechanical interlocking theory will be covered in more detail in the following section.
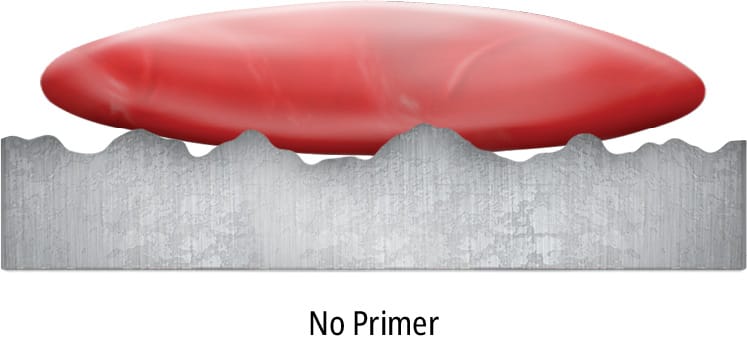

Primers may be applied to a surface by roller, brush, spray or immersion and have variable on-part lives, depending on the chemistry, substrate surface characteristics and environmental influences. Each primer is specifically designed for specific substrates and adhesives, and a primer designed for one chemistry or substrate may not be suitable for another substrate or chemistry.

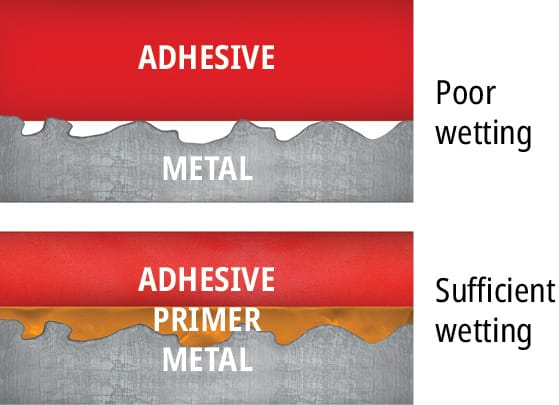
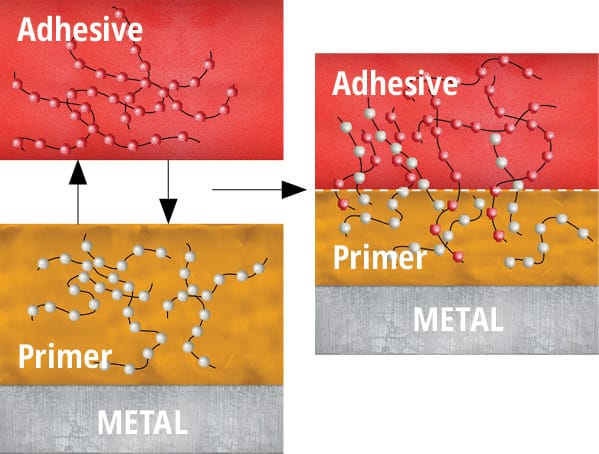

Wetting makes a world of difference
Don’t believe us? Try our free LOCTITE® XPLORE's e-learning and we’ll show you.
Log in and select the chapter Introduction to Bonding and select Prepare to be Amazed to learn more.

